Nitrogen Is A Component Of
Nitrogen in Plants
Healthy plants often contain 3 to 4 percent nitrogen in their higher up-ground tissues. This is a much higher concentration compared to other nutrients. Carbon, hydrogen and oxygen, nutrients that don't play a significant office in most soil fertility management programs, are the only other nutrients present in higher concentrations.
Nitrogen is and so vital because it is a major component of chlorophyll, the chemical compound by which plants utilise sunlight energy to produce sugars from water and carbon dioxide (i.eastward., photosynthesis). It is as well a major component of amino acids, the edifice blocks of proteins. Without proteins, plants wither and die. Some proteins deed as structural units in plant cells while others act as enzymes, making possible many of the biochemical reactions on which life is based. Nitrogen is a component of energy-transfer compounds, such as ATP (adenosine triphosphate). ATP allows cells to conserve and use the energy released in metabolism. Finally, nitrogen is a significant component of nucleic acids such as DNA, the genetic material that allows cells (and eventually whole plants) to abound and reproduce. Without nitrogen, there would be no life equally we know it.
Structure of an Amino Acid
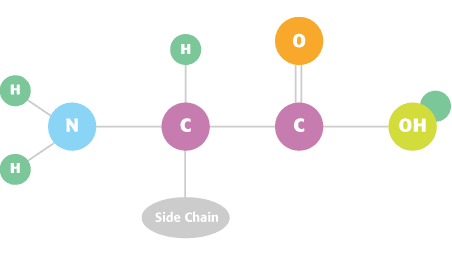
Nitrogen is essential for crops to reach optimum yields. A critical component of amino acids in protein, it also increases protein content of plants directly.
Soil Nitrogen
Soil nitrogen exists in three general forms: organic nitrogen compounds, ammonium (NH₄⁺) ions and nitrate (NO₃⁻) ions.
At any given time, 95 to 99 percent of the potentially available nitrogen in the soil is in organic forms, either in plant and animal residues, in the relatively stable soil organic affair, or in living soil organisms, mainly microbes such as bacteria. This nitrogen is not directly available to plants, but some can be converted to available forms by microorganisms. A very small amount of organic nitrogen may exist in soluble organic compounds, such as urea, that may exist slightly available to plants.
The bulk of establish-available nitrogen is in the inorganic forms NH₄⁺ and NO₃⁻ (sometimes chosen mineral nitrogen). Ammonium ions bind to the soil's negatively charged cation exchange complex (CEC) and behave much similar other cations in the soil. Nitrate ions exercise not bind to the soil solids because they comport negative charges, but exist dissolved in the soil water, or precipitated equally soluble salts under dry conditions.
Natural Sources of Soil Nitrogen
The nitrogen in soil that might eventually exist used by plants has ii sources: nitrogen- containing minerals and the vast storehouse of nitrogen in the atmosphere. The nitrogen in soil minerals is released as the mineral decomposes. This procedure is generally quite slow, and contributes only slightly to nitrogen nutrition on about soils. On soils containing large quantities of NH₄⁺-rich clays (either naturally occurring or developed by fixation of NH₄⁺ added as fertilizer), yet, nitrogen supplied past the mineral fraction may be meaning in some years.
Atmospheric nitrogen is a major source of nitrogen in soils. In the atmosphere, it exists in the very inert N₂ form and must exist converted before information technology becomes useful in the soil. The quantity of nitrogen added to the soil in this fashion is straight related to thunderstorm activity, but well-nigh areas probably receive no more than 20 lb nitrogen/acre per year from this source.
Bacteria such as Rhizobia that infect (nodulate) the roots of, and receive much nutrient energy from, legume plants can ready much more nitrogen per year (some well over 100 lb nitrogen/acre). When the quantity of nitrogen stock-still by Rhizobia exceeds that needed by the microbes themselves, information technology is released for utilise by the host legume plant. This is why well-nodulated legumes do not oft reply to additions of nitrogen fertilizer. They are already receiving enough from the bacteria.
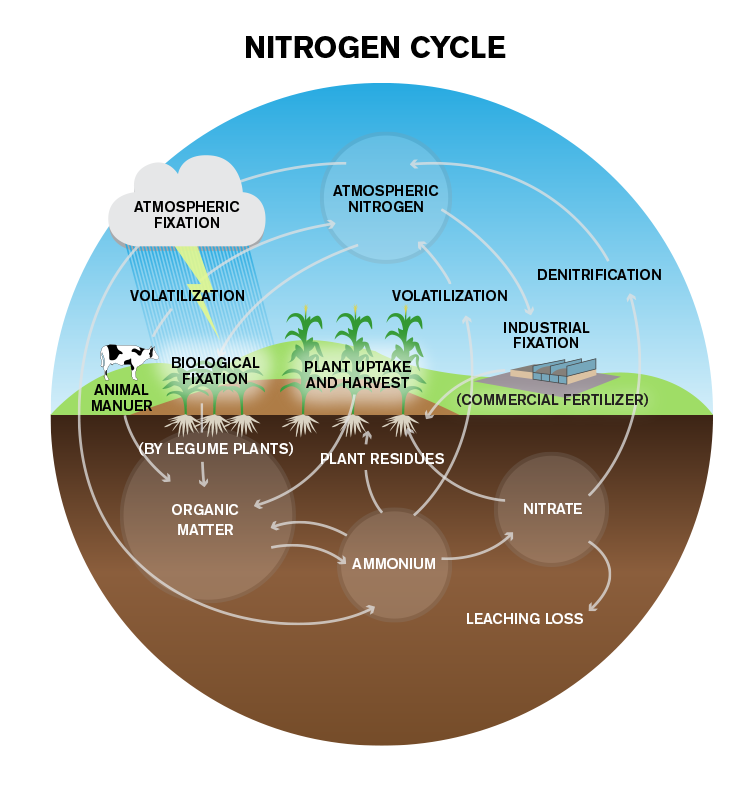
The Nitrogen Cycle
Nitrogen can become through many transformations in the soil. These transformations are frequently grouped into a organisation chosen the nitrogen cycle, which tin be presented in varying degrees of complication. The nitrogen cycle is appropriate for understanding food and fertilizer management. Because microorganisms are responsible for most of these processes, they occur very slowly, if at all, when soil temperatures are below 50° F, but their rates increase apace as soils go warmer.
The heart of the nitrogen bike is the conversion of inorganic to organic nitrogen, and vice versa. Every bit microorganisms grow, they remove H₄⁺ and NO₃⁻ from the soil's inorganic, available nitrogen pool, converting it to organic nitrogen in a process called immobilization. When these organisms dice and are decomposed by others, backlog NH₄⁺ tin can be released dorsum to the inorganic puddle in a process called mineralization. Nitrogen can also be mineralized when microorganisms decompose a material containing more nitrogen than they can use at one time, materials such as legume residues or manures. Immobilization and mineralization are conducted by most microorganisms, and are most rapid when soils are warm and moist, just not saturated with water. The quantity of inorganic nitrogen available for crop utilise ofttimes depends on the amount of mineralization occurring and the residue betwixt mineralization and immobilization.
Ammonium ions (NH₄⁺) not immobilized or taken up chop-chop by higher plants are usually converted speedily to NO₃⁻ ions by a procedure chosen nitrification. This is a two-stride process, during which bacteria chosen Nitrosomonas convert NH₄⁺ to nitrite (NO₂⁻), so other bacteria, Nitrobacter, catechumen the NO₂⁻ to NO₃⁻. This process requires a well-aerated soil and occurs chop-chop enough that one usually finds mostly NO₃⁻ rather than NH₄⁺ in soils during the growing season.
The nitrogen wheel contains several routes by which plant-bachelor nitrogen can be lost from the soil. Nitrate-nitrogen is usually more subject field to loss than is ammonium-nitrogen. Significant loss mechanisms include leaching, denitrification, volatilization and crop removal.
The nitrate course of nitrogen is so soluble that it leaches hands when excess water percolates through the soil. This can be a major loss machinery in coarse-textured soils where water percolates freely, but is less of a problem in finer-textured, more impermeable soils, where percolation is very slow.
These latter soils tend to become saturated easily, and when microorganisms exhaust the gratuitous oxygen supply in the wet soil, some obtain it by decomposing NO₃⁻. In this process, called denitrification, NO₃⁻ is converted to gaseous oxides of nitrogen or to North₂ gas, both unavailable to plants. Denitrification tin cause major losses of nitrogen when soils are warm and remain saturated for more than a few days.
Losses of NH₄⁺ nitrogen are less common and occur mainly past volatilization. Ammonium ions are basically anhydrous ammonia (NH₃) molecules with an actress hydrogen ion (H⁺) attached. When this actress H⁺ is removed from the NH₄ ion by some other ion such equally hydroxyl (OH⁻), the resulting NH₃ molecule can evaporate, or volatilize from the soil. This mechanism is most of import in high-pH soils that contain large quantities of OH⁻ ions.
Ingather removal represents a loss considering nitrogen in the harvested portions of the crop plant is removed from the field completely. The nitrogen in crop residues is recycled back into the system, and is better thought of as immobilized rather than removed. Much is eventually mineralized and may be reutilized past a crop.
Found Nitrogen Needs and Uptake
Plants absorb nitrogen from the soil as both NH₄⁺ and NO₃⁻ ions, but because nitrification is so pervasive in agricultural soils, most of the nitrogen is taken upward every bit nitrate. Nitrate moves freely toward plant roots as they blot water. Once within the plant, NO₃⁻ is reduced to an NH₂ form and is assimilated to produce more than complex compounds. Considering plants require very large quantities of nitrogen, an all-encompassing root system is essential to allowing unrestricted uptake. Plants with roots restricted by compaction may show signs of nitrogen deficiency even when adequate nitrogen is nowadays in the soil.
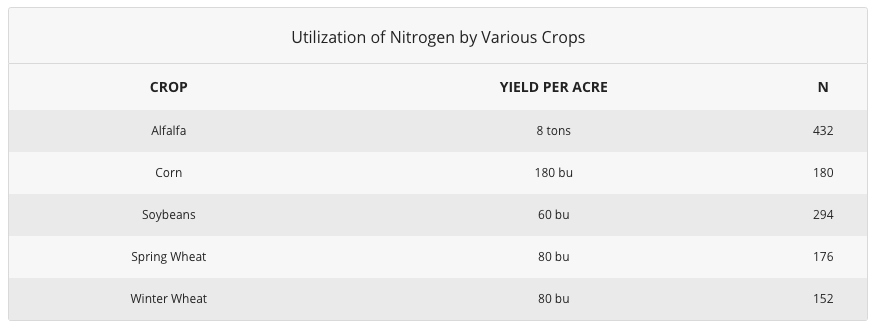
Source: TFI
Most plants accept nitrogen from the soil continuously throughout their lives, and nitrogen demand usually increases as found size increases. A plant supplied with adequate nitrogen grows rapidly and produces large amounts of delicious, greenish foliage. Providing adequate nitrogen allows an almanac crop, such as corn, to abound to full maturity, rather than delaying it. A nitrogen-deficient constitute is generally small and develops slowly considering it lacks the nitrogen necessary to manufacture adequate structural and genetic materials. It is usually pale dark-green or yellow considering it lacks adequate chlorophyll. Older leaves frequently become necrotic and dice as the plant moves nitrogen from less important older tissues to more important younger ones.
On the other manus, some plants may grow so quickly when supplied with excessive nitrogen that they develop protoplasm faster than they can build sufficient supporting cloth in cell walls. Such plants are ofttimes rather weak and may exist prone to mechanical injury. Development of weak straw and lodging of small-scale grains are an case of such an consequence.
Fertilizer Management
Nitrogen Bike
Nitrogen fertilizer rates are determined by the crop to be grown, yield goal and quantity of nitrogen that might be provided by the soil. Rates needed to achieve different yields with different crops vary by region, and such decisions are usually based on local recommendations and experience.
Factors that Determine the Quantity of Nitrogen Supplied by the Soil
-
The quantity of nitrogen released from the soil organic affair
-
The quantity of nitrogen released by decomposition of residues of the previous crop
-
Any nitrogen supplied by previous applications of organic waste
-
Any nitrogen carried over from previous fertilizer applications.
Such contributions can be determined by taking nitrogen credits (expressed in lb/acre) for these variables. For example, corn following alfalfa normally requires less boosted nitrogen than corn following corn, and less nitrogen fertilizer is needed to reach a given yield goal when manure is practical. Equally with rates, credits are usually based on local conditions.
Soil testing is being suggested more ofttimes equally an alternative to taking nitrogen credits. Testing soils for nitrogen has been a useful practice in the drier regions of the Corking Plains for many years, and in that region, fertilizer rates are often adjusted to account for NO₃⁻ establish in the soil prior to planting. In recent years, at that place has been some interest in testing cornfields for NO₃⁻ in the more humid regions of the eastern Usa and Canada, utilizing samples taken in late spring, later on ingather emergence, rather than earlier planting. This strategy, the pre-side-clothes nitrogen soil test (PSNT), has received a great deal of publicity and seems to provide some indication of whether boosted side-dressed nitrogen is needed or not.
Fertilizer Placement
Placement decisions should maximize availability of nitrogen to crops and minimize potential losses. A constitute's roots unremarkably will non grow across the root zone of another establish, and so nitrogen must be placed where all plants have directly access to information technology. Broadcast applications attain this objective. Banding does also when all ingather rows are directly adjacent to a ring. For corn, banding anhydrous ammonia or urea ammonium nitrate (UAN) in alternate row middles is usually as effective as banding in each eye because all rows have access to the fertilizer.
Moist soil conditions are necessary for nutrient uptake. Placement below the soil surface can increase nitrogen availability under dry conditions because roots are more likely to find nitrogen in moist soil with such placement. Injecting side-dressed UAN may produce college corn yields than surface application in years when dry out weather follows side-dressing. In years when rainfall occurs presently after application, subsurface placement is not as disquisitional.
Subsurface placement is usually used to command nitrogen losses. Anhydrous ammonia must exist placed and sealed below the surface to eliminate straight volatilization losses of the gaseous ammonia. Volatilization from urea and UAN solutions can be controlled by incorporation or injection. Incorporating urea materials (mechanically or by rainfall shortly after application) is especially important in no-till situations in which volatilization is aggravated by large amounts of organic material on the soil surface. Applying small amounts of "starter" nitrogen as UAN in herbicide sprays, nonetheless, is usually of trivial concern.
Placing nitrogen with phosphorus often increases phosphorus uptake, particularly when nitrogen is in the NH₄⁺ form and the crop is growing in an alkaline metal soil. The reasons for the result are non completely clear, merely may be due to nitrogen increasing root activity and potential for phosphorus uptake, and nitrification of NH₄⁺ providing acidity, which enhances phosphorus solubility.
Timing of Nutrient Awarding
Timing has a major effect on the efficiency of nitrogen management systems. Nitrogen should be applied to avoid periods of significant loss and to provide adequate nitrogen when the crop needs it well-nigh. Wheat takes up almost of its nitrogen in the jump and early summer, and corn absorbs most nitrogen in midsummer, so ample availability at these times is disquisitional. If losses are expected to be minimal, or can exist effectively controlled, applications before or immediately later planting are effective for both crops. If significant losses, peculiarly those due to denitrification or leaching, are anticipated, split applications, in which much of the nitrogen is applied after crop emergence, can exist effective in reducing losses. Fall applications for corn can be used on well-drained soils, peculiarly if the nitrogen is applied as anhydrous ammonia amended with N-Serve®; all the same, fall applications should exist avoided on poorly drained soils, due to an virtually unavoidable potential for significant denitrification losses. When virtually of a crop's nitrogen supply volition be applied after significant crop growth or positioned abroad from the seed row (anhydrous ammonia or UAN banded in row middles), applying some nitrogen easily attainable to the seedling at planting ensures that the crop will not become nitrogen deficient before gaining access to the main supply of nitrogen.
Minimizing Fertilizer Losses
The major mechanisms for nitrogen fertilizer loss are denitrification, leaching and volatilization. Denitrification and leaching occur under very moisture soil weather condition, while volatilization is most common when soils are only moist and are drying.
Practices for Fugitive Nitrogen Fertilizer Losses
Using an NH₄⁺ source of nitrogen acidifies the soil because the hydrogen ions (H⁺) released during nitrification of the NH₄⁺ are the major cause of acidity in soils. Over fourth dimension, acidification and lowering of soil pH can become meaning.
Nitrogen fertilizers containing NO₃⁻ but no NH₄⁺ make the soil slightly less acidic over time, merely are generally used in much lesser quantities than the others. The acidification due to NH₄⁻ nitrogen is a pregnant factor in the acidification of agricultural fields, only can easily be controlled by normal liming practices.

*A minus sign indicates the number of pounds of calcium carbonate equivalent needed to neutralize the acid formed when 1 ton of the material is added to the soil. (Note that approximately two times this corporeality would exist required if ag-lime is used.) A plus sign indicates the material is basic in nature.
Fertilizing Legumes with Nitrogen
Because the Rhizobia bacteria that infect legume roots normally supply adequate nitrogen to the host plant, well-nodulated legumes rarely respond to additions of nitrogen fertilizer. Occasionally, all the same, soybeans may answer to applications of nitrogen late in the season, presumably because nitrogen fixation in the nodules has declined significantly. Such responses are quite erratic, though, and belatedly-season applications of nitrogen to soybeans are non routinely recommended. The amount of atmospheric nitrogen fixed past non-symbiotic soil organisms varies with soil types, organic thing present and soil pH.
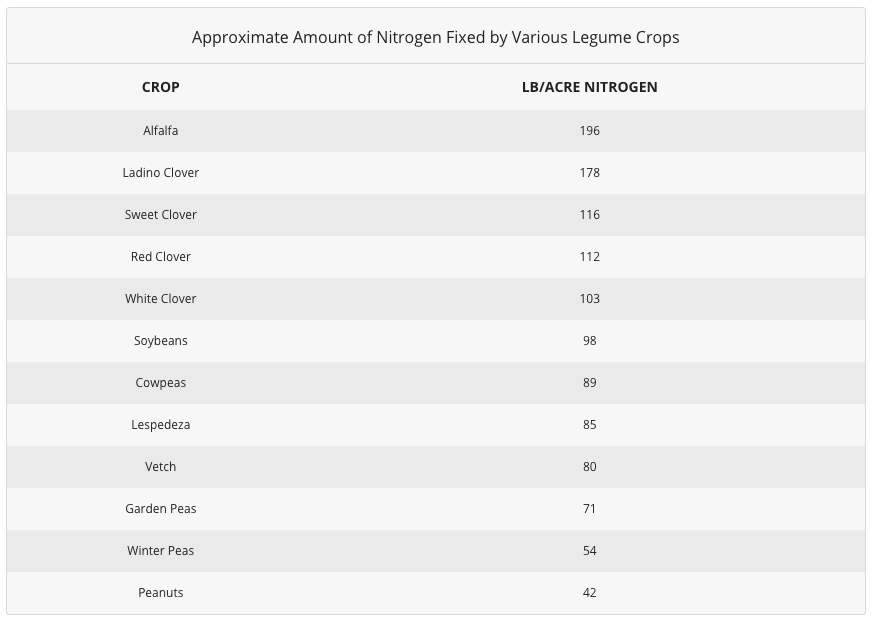
Adapted from "Fertilizers and Soil Amendments" by Follett, Tater and Donahue.
Additives for Nitrogen Fertilizers
N-Serve
N-Serve (nitrapyrin) is a proven material that selectively inhibits one of the bacteria responsible for nitrification. When added to an NH₄⁺ nitrogen material, information technology delays its conversion to NO₃⁻ nitrogen for several weeks. It is most effective when mixed with anhydrous ammonia. This delayed nitrification protects the fertilizer from losses due to denitrification and leaching in seasons when excessive rainfall occurs during the period of inhibition. Using N-Serve is somewhat like buying an insurance policy that pays in years when problems develop.
N-Serve is a registered trademark of Dow AgroScience.
AGROTAIN®
AGROTAIN® (NBPT) is a production that inhibits conversion of urea to ammonium carbonate, thereby reducing the potential for ammonia volatilization from urea materials, including UAN solutions. Like N-Serve®, it might be viewed every bit an insurance policy that will reduce potential nitrogen losses in seasons when tillage or rain does not incorporate the urea into the soil before long after application. AGROTAIN is near useful when urea or UAN is practical without incorporation to the surface of fields with high levels of crop residue, such as in no-till situations, or fields with high pH levels at the surface.
AGROTAIN is a registered trademark of Dow AgroSciences LLC.
Other Additives
Other nitrogen additives available on the market place include ESN® and Instinct®. They can exist used as office of the 4R Nutrient Stewardship strategy to continue nitrogen in its proper place at the fourth dimension the institute needs it. Using nitrogen additive products is considered a all-time direction exercise for nitrogen management.
Instinct is a registered trademark of Dow AgroSciences LLC. ESN is a registered trademark owned by Agrium Inc.
Adjusted from "The Efficient Fertilizer Use Manual",
Nitrogen chapter by Dr. Don Eckert
Nitrogen Is A Component Of,
Source: https://www.cropnutrition.com/nutrient-management/nitrogen
Posted by: busseyfacconly.blogspot.com


0 Response to "Nitrogen Is A Component Of"
Post a Comment