Pb No3 2 Compound Name
| |||
| Identifiers | |||
|---|---|---|---|
| CAS Number |
| ||
| 3D model (JSmol) |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.030.210 | ||
| EC Number |
| ||
| PubChem CID |
| ||
| RTECS number |
| ||
| UNII |
| ||
| UN number | 1469 | ||
| CompTox Dashboard (EPA) |
| ||
| InChI
| |||
| SMILES
| |||
| Properties | |||
| Chemical formula | Pb(NO3)two | ||
| Molar mass | 331.ii thou/mol | ||
| Appearance | colorless or white | ||
| Density | iv.53 g/cmiii | ||
| Melting point | 470 °C (878 °F; 743 K)[2] decomposes | ||
| Solubility in water | 376.5 one thousand/L (0 °C) 597 g/Fifty (25°C) 1270 k/Fifty (100°C) | ||
| Magnetic susceptibility (χ) | −74·ten−vi cmthree/mol[1] | ||
| Refractive index (n D) | 1.782[two] | ||
| Thermochemistry | |||
| Std enthalpy of | −451.ix kJ·mol−1 [1] | ||
| Hazards | |||
| GHS labelling:[4] | |||
| Pictograms |     | ||
| Point give-and-take | Danger | ||
| Hazard statements | H302, H317, H318, H332, H360, H373, H410 | ||
| Precautionary statements | P201, P202, P210, P220, P221, P260, P261, P264, P270, P271, P272, P273, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P310, P312, P314, P321, P330, P333+P313, P363, P370+P378, P391, P405, P501 | ||
| NFPA 704 (burn diamond) | 3 0 1 OX | ||
| Lethal dose or concentration (LD, LC): | |||
| LDLo (lowest published) | 500 mg/kg (guinea pig, oral)[three] | ||
| Safe data sheet (SDS) | ICSC 1000 | ||
| Except where otherwise noted, data are given for materials in their standard land (at 25 °C [77 °F], 100 kPa). Infobox references | |||
Lead(Ii) nitrate is an inorganic compound with the chemical formula Atomic number 82(NOthree)two. It commonly occurs as a colourless crystal or white powder and, unlike well-nigh other lead(Two) salts, is soluble in h2o.
Known since the Centre Ages by the proper noun plumbum dulce, the production of lead(II) nitrate from either metallic lead or lead oxide in nitric acid was small, for direct use in making other lead compounds. In the nineteenth century lead(Ii) nitrate began to be produced commercially in Europe and the United States. Historically, the main use was as a raw material in the product of pigments for lead paints, merely such paints have been superseded by less toxic paints based on titanium dioxide. Other industrial uses included rut stabilization in nylon and polyesters, and in coatings of photothermographic paper. Since around the twelvemonth 2000, lead(II) nitrate has begun to be used in gold cyanidation.
Lead(II) nitrate is toxic and must be handled with care to preclude inhalation, ingestion and skin contact. Due to its hazardous nature, the limited applications of lead(Ii) nitrate are under constant scrutiny.
History [edit]
Lead nitrate was first identified in 1597 past the alchemist Andreas Libavius, who called the substance plumbum dulce, meaning "sweet lead", because of its taste.[5] It is produced commercially by reaction of metallic atomic number 82 with concentrated nitric acid in which it is sparingly soluble.[6] [7] It has been produced every bit a raw material for making pigments such every bit chrome yellowish (pb(II) chromate, PbCrOfour) and chrome orange (basic lead(Two) chromate, Pb2CrO5) and Naples xanthous. These pigments were used for dyeing and press calico and other textiles.[8] It has been used as an oxidizer in blackness powder and together with pb azide in special explosives.[nine]
Production [edit]
Atomic number 82 nitrate is produced by reaction of lead(II) oxide with concentrated nitric acrid:[10]
- PbO + two HNO3(concentrated) → Lead(NOiii)2↓ + H2O
Information technology may also be obtained evaporation of the solution obtained past reacting metallic lead with dilute nitric acid.[11]
- Atomic number 82 + 4 HNO3 → Pb(NO3)2 + 2 NO2 + 2 H2O
Solutions and crystals of lead(II) nitrate are formed in the processing of lead–bismuth wastes from pb refineries.[12]
Structure [edit]
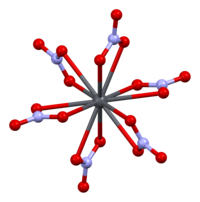
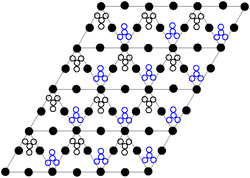
Crystal structure of Pb(NO3)2 [111] plane
The crystal structure of solid lead(II) nitrate has been determined by neutron diffraction.[13] [fourteen] The compound crystallizes in the cubic system with the lead atoms in a face-centred cubic organization. Its space grouping is Pa3Z=4 (Bravais lattice notation), with each side of the cube with length 784 picometres.
The black dots represent the lead atoms, the white dots the nitrate groups 27 picometres above the plane of the lead atoms, and the blue dots the nitrate groups the same distance below this airplane. In this configuration, every lead atom is bonded to twelve oxygen atoms (bond length: 281 pm). All N–O bond lengths are identical, at 127 picometres.[xv]
Research involvement in the crystal structure of atomic number 82(II) nitrate was partly based on the possibility of gratuitous internal rotation of the nitrate groups inside the crystal lattice at elevated temperatures, but this did not materialise.[fourteen]
Chemical properties and reactions [edit]
Solubility of lead nitrate in nitric acid at 26 °C.[xvi]
Lead nitrate decomposes on heating, a property that has been used in pyrotechnics .[ix] It is soluble in water and dilute nitric acrid.
Basic nitrates are formed in when brine is added to a solution. Pbtwo(OH)ii(NO3)2 is the predominant species formed at depression pH. At higher pH Pb6(OH)5(NO3) is formed.[17] The cation [Pb6O(OH)vi]four+ is unusual in having an oxide ion inside a cluster of 3 confront-sharing PbO4 tetrahedra.[18] There is no evidence for the germination of the hydroxide, Atomic number 82(OH)ii, in aqueous solution below pH 12.
Solutions of lead nitrate tin exist used to form co-ordination complexes. Atomic number 82(II) is a hard acceptor; it forms stronger complexes with nitrogen and oxygen electron-donating ligands. For example, combining lead nitrate and pentaethylene glycol (EO5) in a solution of acetonitrile and methanol followed by ho-hum evaporation produced the compound [Pb(NOthree)ii(EO5)].[nineteen] In the crystal structure for this chemical compound, the EOfive chain is wrapped around the lead ion in an equatorial plane similar to that of a crown ether. The 2 bidentate nitrate ligands are in trans configuration. The total coordination number is 10, with the pb ion in a bicapped square antiprism molecular geometry.
The complex formed by lead nitrate with a bithiazole bidentate N-donor ligand is binuclear. The crystal structure shows that the nitrate group forms a bridge between two lead atoms.[xx] One interesting attribute of this blazon of complexes is the presence of a physical gap in the coordination sphere; i.e., the ligands are non placed symmetrically around the metal ion. This is potentially due to a lead lone pair of electrons, also found in lead complexes with an imidazole ligand.[21]
Applications [edit]
Lead nitrate has been used as a heat stabiliser in nylon and polyesters, as a blanket for photothermographic paper, and in rodenticides.[x]
Heating lead nitrate is convenient means of making nitrogen dioxide
In the gilt cyanidation process, addition of lead(Two) nitrate solution improves the leaching procedure. Just express amounts (10 to 100 milligrams pb nitrate per kilogram golden) are required.[22] [23]
In organic chemistry, it may be used in the preparation of isothiocyanates from dithiocarbamates.[24] Its use as a bromide scavenger during SN1 commutation has been reported.[25]
Safety [edit]
Lead(II) nitrate is toxic, and ingestion may pb to acute pb poisoning, as is applicative for all soluble atomic number 82 compounds.[26] All inorganic lead compounds are classified past the International Agency for Research on Cancer (IARC) as probably carcinogenic to humans (Category 2A).[27] They have been linked to renal cancer and glioma in experimental animals and to renal cancer, encephalon cancer and lung cancer in humans, although studies of workers exposed to lead are often complicated by concurrent exposure to arsenic.[28] Lead is known to substitute for zinc in a number of enzymes, including δ-aminolevulinic acrid dehydratase (porphobilinogen synthase) in the haem biosynthetic pathway and pyrimidine-five′-nucleotidase, important for the correct metabolism of DNA and can therefore crusade fetal harm.[29]
References [edit]
- ^ a b CRC handbook of chemistry and physics : a fix-reference book of chemical and physical data. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: others (link) - ^ a b Patnaik, Pradyot (2003). Handbook of inorganic chemicals. New York: McGraw-Hill. p. 475. ISBN0-07-049439-viii. OCLC 50252041.
- ^ "Atomic number 82 compounds (as Pb)". Immediately Dangerous to Life or Wellness Concentrations (IDLH). National Found for Occupational Safety and Health (NIOSH).
- ^ "Lead nitrate". pubchem.ncbi.nlm.nih.gov . Retrieved 19 December 2021.
- ^ Libavius, Andreas (1595). Alchemia Andreæ Libavii. Francofurti: Iohannes Saurius.
- ^ Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. Vol. 16 (11th ed.). Cambridge University Printing. pp. 314–320.
- ^ Macgregor, John (1847). Progress of America to year 1846. London: Whittaker & Co. ISBN0-665-51791-2.
- ^ Partington, James Riddick (1950). A Text-book of Inorganic Chemistry. MacMillan. p. 838.
- ^ a b Barkley, J. B. (October 1978). "Lead nitrate as an oxidizer in blackpowder". Pyrotechnica. Post Falls, Idaho: Pyrotechnica Publications. four: 16–xviii.
- ^ a b Greenwood, Norman North.; Earnshaw, A. (1997). Chemical science of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. pp. 388, 456. ISBN0-7506-3365-4.
- ^ Othmer, D. F. (1967). Kirk-Othmer Encyclopedia of Chemic Applied science. Vol. 12 (Atomic number 26 to Manganese) (second completely revised ed.). New York: John Wiley & Sons. p. 272. ISBN0-471-02040-0.
- ^ "Product catalog; other products". Tilly, Belgium: Sidech. Archived from the original on 2007-07-01. Retrieved 2008-01-05 .
- ^ Hamilton, West. C. (1957). "A neutron crystallographic study of pb nitrate". Acta Crystallogr. 10 (2): 103–107. doi:ten.1107/S0365110X57000304.
- ^ a b Nowotny, H.; K. Heger (1986). "Structure refinement of lead nitrate". Acta Crystallogr. C. 42 (2): 133–35. doi:10.1107/S0108270186097032.
- ^ "Cấu trúc của chì nitrat". Retrieved 15 July 2019.
- ^ Ferris, L. M. (1959). "Atomic number 82 nitrate—Nitric acid—H2o organization". Journal of Chemical & Engineering Data. 5 (3): 242. doi:10.1021/je60007a002.
- ^ Pauley, J. L.; M. K. Testerman (1954). "Bones Salts of Pb Nitrate Formed in Aqueous Media". Periodical of the American Chemical Social club. 76 (16): 4220–4222. doi:x.1021/ja01645a062.
- ^ Greenwood, Norman North.; Earnshaw, Alan (1997). Chemistry of the Elements (second ed.). Butterworth-Heinemann. ISBN978-0-08-037941-8. p. 395
- ^ Rogers, Robin D.; Andrew H. Bail; Debra M. Roden (1996). "Structural Chemistry of Poly (ethylene glycol). Complexes of Lead(II) Nitrate and Lead(2) Bromide". Inorg. Chem. 35 (24): 6964–6973. doi:x.1021/ic960587b. PMID 11666874.
- ^ Mahjoub, Ali Reza; Ali Morsali (2001). "A Dimeric Mixed-Anions Atomic number 82(II) Complex: Synthesis and Structural Characterization of [Lead2(BTZ)4(NO3)(H2O)](ClO4)3 {BTZ = 4,iv'-Bithiazole}". Chemistry Letters. thirty (12): 1234. doi:x.1246/cl.2001.1234.
- ^ Shuang-Yi Wan; Jian Fan; Taka-aki Okamura; Hui-Fang Zhu; Xing-Mei Ouyang; Wei-Yin Sun & Norikazu Ueyama (2002). "2D four.82 Network with threefold parallel interpenetration from nanometre-sized tripodal ligand and atomic number 82(Two) nitrate". Chem. Commun. (21): 2520–2521. doi:10.1039/b207568g.
- ^ Habashi, Fathi (1998). "Recent advances in gilded metallurgy". Revisa de la Facultad de Ingeniera, Universidad Fundamental de Venezuela. 13 (2): 43–54.
- ^ "Auxiliary agents in gold cyanidation". Gilded Prospecting and Gold Mining. Retrieved 2008-01-05 .
- ^ Dains, F. B.; Brewster, R. Q.; Olander, C. P. "Phenyl isothiocyanate". Organic Syntheses. ; Commonage Volume, vol. i, p. 447
- ^ Rapoport, H.; Jamison, T. (1998). "(S)-N-(9-Phenylfluoren-9-yl)alanine and (S)-Dimethyl-N-(9-phenylfluoren-9-yl)aspartate". Organic Syntheses. ; Collective Volume, vol. nine, p. 344
- ^ "Lead nitrate, Chemic Safety Carte du jour 1000". International Labour Organisation, International Occupational Condom and Health Information Centre. March 1999. Retrieved 2008-01-nineteen .
- ^ "Inorganic and Organic Atomic number 82 Compounds" (PDF). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer. Suppl. 7: 239. 1987. Archived from the original (PDF) on 2008-03-06. Retrieved 2008-01-xix .
- ^ World Wellness Organization, International Agency for Research on Cancer (2006). "Inorganic and Organic Atomic number 82 Compounds" (PDF). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. International Bureau for Research on Cancer. 87. ISBN92-832-1287-8. Archived from the original (PDF) on 2007-10-21. Retrieved 2008-01-01 .
- ^ Mohammed-Brahim, B.; Buchet, J.P.; Lauwerys, R. (1985). "Erythrocyte pyrimidine 5'-nucleotidase activity in workers exposed to lead, mercury or cadmium". Int Curvation Occup Environ Health. 55 (three): 247–52. doi:ten.1007/BF00383757. PMID 2987134. S2CID 40092031.
External links [edit]
- Woodbury, William D. (1982). "Lead". Mineral Yearbook Metals and Minerals. Bureau of Mines: 515–42. Retrieved 2008-01-eighteen .
- "Lead". NIOSH Pocket Guide to Chemical Hazards. National Institute for Occupational Condom and Health. September 2005. NIOSH 2005-149. Retrieved 2008-01-nineteen .
- "Atomic number 82 and Lead Compounds Fact Sheet". National Pollutant Inventory. Australian Government, Department of the Environment and Water Resources. July 2007. Archived from the original on Jan eleven, 2008. Retrieved 2008-01-nineteen .
- "Atomic number 82". A Healthy Home Environment, Wellness Hazards. United states Brotherhood for healthy homes. Archived from the original on 2008-02-20. Retrieved 2008-01-19 .
- Cloth Safe Data Sheets
- MSDS for atomic number 82 nitrate, PTCL, Oxford University
- MSDS for lead nitrate, Scientific discipline Stuff Inc
- MSDS for lead nitrate, Iowa Country Academy
Pb No3 2 Compound Name,
Source: https://en.wikipedia.org/wiki/Lead%28II%29_nitrate
Posted by: busseyfacconly.blogspot.com



![{\displaystyle {\ce {2 Pb(NO_3)_2->[\Delta]2PbO + 4NO_2 +O_2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/06f64696eaa883432008d7e6af4c5af9b60a6b85)

0 Response to "Pb No3 2 Compound Name"
Post a Comment